Abstract
The Ediacaran period (635–541 Ma) was a time of major environmental change, accompanied by a transition from a microbial world to the animal world we know today. Multicellular, macroscopic organisms preserved as casts and molds in Ediacaran siliciclastic rocks are preserved worldwide and provide snapshots of early organismal, including animal, evolution. Remarkable evolutionary advances are also witnessed by diverse cellular and subcellular phosphatized microfossils described from the Doushantuo Formation in China, the only source showing a diversified assemblage of microfossils. Here, we greatly extend the known distribution of this Doushantuo-type biota in reporting an Ediacaran Lagerstätte from Laurentia (Portfjeld Formation, North Greenland), with phosphatized animal-like eggs, embryos, acritarchs, and cyanobacteria, the age of which is constrained by the Shuram–Wonoka anomaly (c. 570–560 Ma). The discovery of these Ediacaran phosphatized microfossils from outside East Asia extends the distribution of the remarkable biota to a second palaeocontinent in the other hemisphere of the Ediacaran world, considerably expanding our understanding of the temporal and environmental distribution of organisms immediately prior to the Cambrian explosion.
Similar content being viewed by others
Introduction
Remarkably detailed preservation of cells and soft tissues has been described from several Precambrian Lagerstätten, providing some of the best documented examples of early organismal evolution1,2,3. Typically, this quality of preservation is made possible through several pathways, of which diagenetic phosphate replacement of originally organic material (“Doushantuo-type preservation”) has provided some of the most spectacular descriptions of putative animal embryos, acritarchs, and small shelly fossils across the Ediacaran–Cambrian boundary4,5,6. Following their discovery and rise to fame in the late 1990s, the fossils from the well-known Ediacaran Doushantuo Formation of China have sparked controversy and debate, especially regarding speculative interpretation as fossil animal embryos5,7,8,9,10,11,12,13. However, in contrast to the widespread localities yielding macroscopic assemblages, sites yielding Doushantuo-type microscopic assemblages, which could help to resolve some of the most fundamental questions on the evolution of life and clarify the distribution of these organisms in the Ediacaran world, have proved elusive14.
Here we present the first record of Ediacaran Doushantuo-type microfossils from Laurentia (Portfjeld Formation, North Greenland). The Portfjeld biota consists of three-dimensionally preserved putative eggs and embryos, as well as acanthomorphic and leiosphaeric acritarchs, red algal thalli, sheet-like and oscillatoriacean cyanobacteria, and microbial mat fragments. The assemblage is directly comparable to similarly preserved fossils from the Doushantuo Formation but its significance at this time lies in greatly expanding the known record of Ediacaran phosphatized microfossils geographically, from the northern hemisphere of the Ediacaran world into the middle latitudes of its southern hemisphere15,16. In addition, the preservation of the Portfjeld biota in a shallow water setting greatly increases our insight into the environments where life evolved during the Ediacaran.
Results and discussion
Stratigraphic context of the Portfjeld Formation
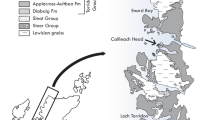
The Portfjeld Formation is the lowermost formation of the Franklinian Basin in southern Peary Land (Figs. 1 and 2a, b), resting unconformably on Mesoproterozoic sandstones of the Independence Fjord Group and localized erosionally truncated outliers of Neoproterozoic tillites and associated carbonates of inferred Marinoan affinity (for regional stratigraphic reviews, see17,18). The carbonate-dominated Portfjeld Formation is overlain, at a karstified unconformity (Fig. 2c), by transgressive fluvial to marine shelf siliciclastics of the Buen Formation. The sandstone-dominated lower member of the Buen Formation yields trace fossils of early Cambrian age19, while the mudstone-dominated upper member contains rich faunas of Cambrian Series 2 (Stage 3–4) age20.
a Portfjeld Formation—basal Buen sandstones at Midsommersøer, notice the conspicuous band of dark cherty dolomites. b Detail of Portfjeld Formation, west of Midsommersøer, with the same darky cherty dolomites overlain by thrombolitic mounds, showing the lithostratigraphic horizon yielding the Portfjeld biota. c View looking east along Wandel Dal with Midsommersøer, taken from the fossil locality (off shot left). Scale bar valid for a.
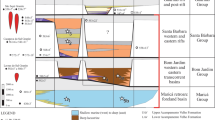
a Geological map showing the sample locality at Midsommersøer, North Greenland. b Stratigraphic schemes in northern Ellesmere Island and North Greenland. c Stratigraphic section through the Portfjeld Formation at the western end of Midsommersøer compared with δ13Ccarb (o/oo PDB) values indicating the Shuram–Wonoka anomaly (about 570–560 Ma), “F” indicates the sample locality.
The Portfjeld Formation comprises two discrete stratigraphic packages separated by a regionally developed karstic unconformity. The lower succession, about 170-m thick, is dominated by dolostones with rare limestones and represents two transgressive–regressive cycles of a carbonate ramp. Typical facies include hummocky cross-stratified intraclast-rich grainstones and cherty dark dolostones of the mid- and outer ramp, and ooid–pisoid grainstones and varied microbial facies of the inner ramp, including columnar and meter-scale domal stromatolites and thrombolitic bioherms. The capping hiatal surface shows penetrative and multi-generational karstic features extending some 40 m beneath the surface (Fig. 2c), including extensive interstratal solution, brecciation, and successive cave/vug/fracture fills and cementation, testifying to a protracted period of subaerial exposure. The transgressive succession of the upper Portfjeld Formation (c. 70–90 m thick) comprises fluvial sandstones and mudstones succeeded by high-energy shallow marine carbonate and siliciclastic facies, truncated upwards by dolines and karstic collapse structures at the Portfjeld–Buen formation boundary.
Chemostratigraphy shows that the δ13C of the Portfjeld Formation carbonate samples range from +4‰ to −8‰. Positive δ13C values persist over the lower c. 40 m of the formation, before a marked negative shift of 12‰ down to values of −8‰. A more gradual increase characterizes the δ13C values up-section through the karstified strata to the karstic unconformity at 167 m, following which there is a clear stabilization in δ13C values to values around 0 to −1‰ for the remainder of the succession (Fig. 2c).
The δ13C database for Neoproterozoic carbonate sections has proliferated within the last 30 years to the point where a δ13C compilation curve can act as a chemostratigraphic correlation tool for newly studied sections. Utilizing chemostratigraphy as a chronology tool involves the correlation of globally coherent geochemical perturbations and trends in vertical carbonate successions, within a broadly understood timeframe. This is particularly useful when attempting to refine age estimates for successions that lack abundant biostratigraphical and/or radiometric data. Utilizing the most up-to-date δ13C chemostratigraphic framework21, the asymmetric negative δ13C excursion and more gradual recovery displayed by the mid-section of the Portfjeld Formation can be aligned with the most extreme C-isotope variation recorded in Earth’s history: the Shuram–Wonoka anomaly. The form and magnitude (∼12‰) of this δ13C excursion, as well as a nadir value of −8‰, are unique to the Shuram–Wonoka anomaly and deter its alignment with other Neoproterozoic excursions, as well as the Basal Cambrian Isotope Excursion, BACE21. The Shuram–Wonoka anomaly is recognized intercontinentally in Late Ediacaran strata22 and provides a broad chronostratigraphic marker to constrain the biostratigraphy presented in this study. Williams and Schmidt22 noted that the Wonoka excursion spanned an interval of up to 10 Myr. from about 570 to 560 Ma and was recognized in shallow marine shelf environments on three palaeocontinents with low palaeolatitudes (<32°), whereas the North Greenland record reported here is from middle palaeolatitudes15,16.
The Portfjeld biota
Well-preserved, phosphatized spiral oscillatoriacean cyanobacteria were recovered from strata in southern Peary Land and described by Peel23 (Fig. 2c, locality F). That study also noted the presence of smooth, wrinkled, or crumpled spheres resembling Olivooides, which prompted the present investigation. Consequently, we processed new fractions of the stromatolitic dolostone sample from the Portfjeld Formation. Here, we report some of the main findings within a diverse assemblage of microfossils that is comparable to the long-studied and highly important Doushantuo Formation biota of China.
Undisputed animal embryos were first identified from the Cambrian of China through the description of a series of developmental stages in Olivooides and Markuelia. Despite being simple in morphology there is good evidence from developmental series showing that at least some Olivooides develop into cnidarians4,24,25 but the simple spherical morphology of these earliest growth stages also permits other interpretations (e.g., echinoderms8). Markuelia is usually considered to be a scalidophoran26. Similarly, proposed animal cleavage embryos have been reported also from the older Ediacaran Doushantuo Formation, but their interpretation is contested with several hypotheses concerning their affinity still current5,9,10,12. The putative eggs and embryos described here are directly comparable in morphology and age to those from the Doushantuo Formation.
Biologically, the transition from egg to embryo comes at fertilization, after which the egg enters the reproductive stage. In fossil material this distinction is normally seen as a ball of cells, where the number of cells doubles during each division. Spheroidal microfossils with smooth envelopes recovered from Portfjeld Formation are interpreted as putative eggs. The embryo-like fossils from the Portfjeld biota consists of clusters (150–170 µm in diameter) of hundreds of individual cells, normally 15–20 µm in diameter, but many are smaller (5–10 µm in diameter). The cells are tightly packed and seem to extend inwards. Neighboring cells appear to accommodate each other, indicating that they are not rigid algal clusters. Most cells are complete but show evidence of deflation or, where broken, display internal phosphatized contents. One specimen (Fig. 3a, b) is interpreted as late stage “Megaclonophycus-stage” (compare Fig. 2G in27). Two others (Fig. 3c–f) are similar to cleavage embryos (256-cell or similar) reported from the Cambrian Kuanchuanpu Formation in China (compare Fig. 5 in28) but comparisons can also be made with Wengania globosa and Wengania exquisita from the Ediacaran Weng’an biota29. A morphological furrow may be present. A peanut-shaped specimen (Fig. 3i) can be compared with the germinating stage in Tianzhushania10, although this simple morphology is not sufficient in itself to make such a definite link. Taxonomic details have been examined in various contexts with regards to suites of developmental stages (for example referring all developmental stages including Megasphaera, Parapandorina, Megaclonophycus, and Yintianzhushania to Tianzhushania) and we refrain from commenting further on this here, but see12,30,31,32 for discussion. Many vesicles are hollow and show evidence of flexible deformation during deflation prior to phosphatization (Fig. 3h, k). Others, which are more delicate, break during mounting to reveal the originally organic internal contents (Fig. 4g). Many other smooth vesicles show evidence for pre-determined rupture (Fig. 3j, m). Simple, lobose, pseudoparenchymatous thalli (Fig. 3g) resembling florideophyte red algae (Gremiphyca corymbiata)29 are also present in the Portfjeld biota.
a–f Putative cleavage embryos. b, d, f Enlarged to show the detail of polygonal cell junctions. g Red algal thalli similar to Gremiphyca corymbiata. h–m Putative eggs showing various degrees of taphonomic degradation (h, k ductile; j, m brittle). i Shows a possible peanut-shaped cell division. j, m Showing breakage of vesicle wall, the shape of the breakage, its size and location on the specimen is similar in many specimens and may therefore be interpreted as a biological feature rather than random breakage. n Shows a comparably large unidentified acritarch showing polygonal shrinkage and a golf ball-like vesicle surface structure. Scale bar 100 µm, unless where individually stated. Accession numbers; a, b PMU 36863/1. c, d PMU 36864/1. e, f PMU 36865/1. f PMU 36865/1. g PMU 36866/1. h PMU 36867/1. i PMU 36868/1. j PMU 36869/1. k PMU 36869/2. l PMU 36870/1. m PMU 36869/3. n PMU 36864/2.
a, b Cavaspina acuminata, showing nature of sparsely separated, tapering processes. c, d Asterocapsoides wenganensis showing densely arranged, conical processes. Box in d shows the conical shape of mostly unbroken processes. e, f Helically anti-clockwise coiling, spheroidal microfossils with closed termination. g Putative egg shell broken during preparation displaying internal contents (indicated by arrow). h Microbial mat displaying community structures with intertwined filamentous structures. i, j Coiled cyanobacterium Jiangispirellus groenlandicus and close up of individual cell walls. k Spirellus shankari cyanobacterium showing the nature of the coiling helix. Scale bar 100 µm, unless where individually stated. Accession numbers; a, b PMU 36871/1. c, d PMU 36872/1. e PMU 36866/2. f PMU 36866/3. g PMU 36872/2. h PMU 36873/1. i, j PMU 36873/2. k PMU 36874/1.
In addition to fossils previously interpreted as eggs and embryos, acanthomorphic acritarchs are rare but well-preserved and important constituents of the Portfjeld biota. Cavaspina acuminata (Fig. 4a, b) is a spheroidal vesicle ~160 µm in diameter with solid and widely separated processes tapering to a conical tip that seems to curve. About 40 processes, many of which are broken, are visible on the vesicle surface (unbroken processes ~15 µm long, which is 10% of vesicle diameter, width at base 5 µm). The originally spheroidal (long axis ~185 µm) Asterocapsoides wenganensis (Fig. 4c, d) is characterized by its short, hollow, homomorphic, evenly distributed, conical processes that taper to a sub-rounded tip (processes are 15–20 µm long, 10–15 µm at base and 5 µm at the tip, and spaced 5 µm apart). Similar acanthomorphic acritarchs (e.g., Mengeosphaera with biform processes, or Meghystrichosphaeridium, with pentagonal or hexagonal fields around the processes) are described from Doushantuo, displaying a taphonomic and taxonomic connection between the Portfjeld and the Doushantuo biota. Spheroidal vesicles, slightly compressed at the poles (diameter 160–180 and 210–300 µm, individual whorls ~50–60 µm in thickness), consisting of three, anti-clockwise coiling whorls, with a rounded termination are rare but well-preserved, and indicative of early biological chirality (Fig. 4e, f). Similar fossils, although larger and with clockwise coiling, were described as the later-stage part of a developmental series (compare Fig. 1O in33)13.
Helically coiled filamentous microfossils are very common remains in the Portfjeld biota; they are also well represented in the Khesen Group of Mongolia14, but rare in the Doushantuo Formation. Three main types, Obruchevella, Spirellus, and Jiangispirellus, all interpreted as oscillatoriacean cyanobacteria, were initially described23. Jiangispirellus groenlandicus (Fig. 4i, j) consists of an open-coiled trichome with delicately preserved cell structure. Spirellus shankari (Fig. 4k) consists of a helix without evidence of cell structure and is interpreted as a filament that is often calcified (now phosphatized). The different types of cyanobacteria show a range in differential taphonomic preservation representing degrees of degradation and mineralization of the original form.
Evolutionary importance of the Portfjeld biota
Peel23 considered the oscillatoriacean cyanobacteria within the Portfjeld biota to be consistent with an early Cambrian age following geological correlation with the Ella Bay Formation of easternmost Ellesmere Island (Nunavut, Canada; Fig. 2b), where samples with early Cambrian macrofossils were known at a lower stratigraphic level34. However, the fossils reported by Long34 from below the Ella Bay Formation, the direct lithological correlative of the Portfjeld Formation along the northern coast of Greenland, were demonstrated subsequently to have been tectonically emplaced from overlying strata of the Cambrian Ellesmere Group35 (Fig. 2b). In consequence, prior to the present discoveries, biostratigraphic control of the age of the Ella Bay and Portfjeld formations was restricted to early Cambrian fossils and trace fossils occurring above the formations in Ellesmere Island and North Greenland18,20,35. A late Neoproterozoic age for the Portfjeld and Ella Bay formations was proposed tentatively by Dewing et al.35 on the basis of correlation with the Risky Formation of the Mackenzie Mountains, northwestern Canada, and the Spiral Creek Formation of North-East Greenland. The age of the former is constrained by Ediacaran macrofossils36, whereas the Spiral Creek Formation is a correlative of successions in eastern Svalbard yielding Neoproterozoic acritarchs37.
The δ13C values in the uppermost carbonates of the Portfjeld Formation, above the karstic unconformity, are compatible with global early Cambrian values. This confirms the interpretation that the intra-Portfjeld unconformity represents a substantial depositional hiatus, supporting the view that the Portfjeld Formation spans the Precambrian–Cambrian boundary18.
Simple multicellular organisms may have evolved already in the Mesoproterozoic38 but it was first in the Ediacaran that complex eukaryotes began to diversify. Until now, the Doushantuo Formation has been our main source of information on soft-bodied organisms predating the classical and enigmatic, macroscopic Ediacaran biota12,39. As such, the discovery of the same type of fossils from North Greenland offers important additional evidence to understanding soft-bodied organismal evolution. The many spheroidal fossils (Fig. 5) discovered in the Portfjeld biota show a variety of morphologies consistent with interpretations that conform well with both blastula stage embryos and spiral stage embryos13. The exact phylogenetic framework is complex; multicellularity, for example, evolved on many different occasions and independently in animals, fungi, and algae40,41. Modern animal embryos or volvocine green algae may provide analogs to Ediacaran embryos but these interpretations are nevertheless imperfect; it is unlikely that the fossils described here represent crown-group animals. The Portfjeld acritarchs form part of a globally distributed and diverse assemblage of morphologically complex and well-documented acritarchs described from carbonaceous compressions in shales, from thin-sectioned cherts as well as phosphatized42,43,44,45,46,47,48. As with the eggs and embryos, phylogenetic uncertainties must be resolved through study of available material from all assemblages.
a Putative cleavage embryo displaying individual cells. b–s Putative eggs/embryos in various states of degradation; internal contents are often preserved and seen as external enveloping layer is broken or peeled off (b–d, g–i, q) but absent when compressed (p); surface structures vary from golf ball-like (f), wrinkled (b), pitted (j) to smooth (h), and from thin-, (s) to thick-walled (g). Some specimens have surface structures that may represent grooves (arrow in n) or polar invaginations (arrows in g). t Probable tightly coiled cyanobacterium (but see also Fig. 6 in Yin et al.13 for similar pseudo-uncoiling in Helicoforamina). Scale bar 100 µm. Accession numbers; a PMU 36868/2. b PMU 36869/4. c PMU 36874/2. d PMU 36875/1. e PMU 36876/1. f PMU 36876/2. g PMU 36877/1. h PMU 36875/2. i PMU 36877/2. j PMU 36877/3. k PMU 36874/3. l PMU 36869/5. m PMU 36876/3. n PMU 36872/2. o PMU 36878/1. p PMU 36864/3. q PMU 36865/2. r PMU 36879/1. s PMU 36877/4. t PMU 36868/2.
The Portfjeld biota seems to preserve specimens that are generally smaller (~100–200 µm) than their morphologically similar Doushantuo biota counterparts (~400–500 µm or larger). There may be several reasons for this discrepancy but they all fall within the framework of natural variation. For the embryo-like fossils, ontogeny may play a role but more importantly the geographic distance between Portfjeld and Doushantuo and the differences in their environments of accumulation must also be taken into consideration.
Palaeogeographically, South China (including the Yangtze Block and the Doushantuo Formation) was probably drifting southwards from a low northerly latitude at the time of deposition of the Weng’an biota, usually suggested to be ~580 Ma ago49,50, although it could be as old as 609 ± 5 Ma51. Laurentia (including North Greenland and the Portfjeld Formation) is estimated to have lain at palaeolatitudes of 30–75° S, with Laurentia completely isolated from all other continents (Fig. 6). Thus, Laurentia and South China were significantly separated from each other at the time of deposition of the two biotas, lying in different hemispheres. While the Weng’an biota yields the oldest putative metazoans, the discovery of similar fossils from the Portfjeld Formation, half a world away, demonstrates that these early possible animals had a worldwide distribution. The palaeogeographic separation is evident but not surprising given the global distribution of many other important Ediacaran fossils. It is therefore perhaps a question of propitious preservation rather than geographic constraints. The two biotas are seemingly older than most of the classic, enigmatic macrofossil biotas now known globally (Fig. 6), with the potential exception of the Avalon biota (574–564 Ma52). Furthermore, they occupied different environments with the Portfjeld biota deposited in an inner carbonate shelf environment18, whereas the Weng’an biota accumulated on the outer shelf39,53. The driving force behind this early evolution has often been attributed to ocean oxidation and fluctuations in the marine carbon and sulfur cycles, but the successful establishment of these early ecosystems may have been dependent on local environmental fluctuations54. However, the discovery of the Portfjeld biota indicates that this early evolution was not restricted locally to China, nor to outer shelf environments, but flourished in geographically separated areas, as was the case also with the younger macrobiota as well as the acanthomorphic acritarchs ranging throughout the Ediacaran. Thus, oxygenation was probably widespread at this time, providing new direct evidence about the early evolution of cellularly differentiated eukaryotes and even the early evolution of animals.
a Stratigraphic distribution of the Portfjeld biota and the Weng’an biota (representative of Doushantuo Formation), the latter of which predates the classical macroscopic “Ediacaran biota” and probably also predating the Gaskiers glaciation39. The potential stratigraphic range of the biotas is indicated by the vertical line but detailed stratigraphic correlation and relationships to other biotas remain to be resolved. b Palaeogeographic reconstruction at ca. 580 Ma, black polygons show location of the two taphonomic windows of this type known from the Ediacaran and their spatial separation (adapted from49,54).
It is evident that the Portfjeld biota predates the nadir of the Shuram–Wonoka anomaly (Fig. 2c). However, there is no geochemical or lithostratigraphic evidence in the measured Portfjeld section to suggest that this succession encompasses the Gaskiers Glaciation (580 Ma)55, indicating that Portfjeld biota, age wise, lies between the Shuram–Wonoka anomaly and the Gaskiers Glaciation (Fig. 5a) and likely preserves a different evolutionary scenario. Published age estimates for the Weng’an biota range from ~580 Ma ago49,50 to 609 ± 5 Ma51 and suggest that the Weng’an biota may be at least 10 Ma or possibly as much as 40 Ma older than the Portfjeld biota. Given the magnitude of the age difference, however uncertain, the degree of palaeogeographic separation of the localities and their contrasting environments, it is evident that the Portfjeld biota provides an additional window onto the early evolution of Ediacaran life, rather than a mere duplication of the Weng’an event.
Conclusions
The Portfjeld Formation crops out over hundreds of kilometers in North Greenland but is poorly known on account of its remoteness. The assemblage of extremely well-preserved microfossils presented here, and its striking similarity to previously described fossils from the Doushantuo Formation of China, demonstrates greater complexity and worldwide distribution of the late Ediacaran ecosystem than previously recognized. The finds from North Greenland extend the known distribution of the Ediacaran Doushantuo-like biota along the length of the Pannotian palaeocontinent, from low to middle latitudes in the northern hemisphere (China) to the middle latitude position in the southern hemisphere occupied by North Greenland in eastern Laurentia; their age is confirmed by chemostratigraphy.
With a background in the largely unexplored potential represented by the Portfjeld Formation, the new discoveries offer excellent prospects for resolving the phylogenetic relationships of many of these problematic multicellular Ediacaran eukaryotes and a better understanding of the environments in which they evolved.
Methods
Material
The fossiliferous sample was collected by John S. Peel and Peter Frykman in 1978 from the lower succession, about 50 m above the base of the formation (equivalent to c. 50 m on Fig. 1c) on the north side of Wandel Dal, west of Øvre Midsommersø (82°14’ N, 36°06’ W, Fig. 1). Samples for stable isotope geochemistry were collected in 2006 at c. 5 m intervals throughout the carbonate intervals of the formation. All collection activities occurred within the Danish State and no international transport or permits were required.
Stromatolitic dolostone rock fragments from sample 271770, a split of the original sample 271769 processed by Peel23, were macerated in weak acetic or formic acid and sieved in fractions down to 44 µm. The residues containing phosphatized microfossils were hand-picked under a binocular microscope. Specimens were mounted on aluminium stubs and imaged under high power using a Zeiss Supra 35VP scanning electron microscope. Images were cropped using Affinity Photo and Affinity Design.
Geochemistry/isotopes
Carbon isotopes were analyzed at the Department of Geography and Geology, University of Copenhagen, using a Micromass mass spectrometer equipped with a multiflow gas bench inlet preparation system. CO2 was liberated by reaction with purified phosphoric acid at 80 °C. Calibrations to V-PDB standard via NBS-19 were performed using the Copenhagen in-house marble standard (LEO). Reproducibility of replicated standards is better than 0.1‰ for δ13C. Data are reported using the conventional δ notation to indicate the ‰ deviation from the arbitrary V-PDB (Vienna-Pee Dee Belemnite) standard.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All imaged fossil material is deposited at the Paleontological collections of Museum of Evolution (PMU) Uppsala University, Sweden. Accession numbers are provided for each fossil using a PMU number. Geochemical datasets are available upon request by contacting Robert Frei (robertf@ign.ku.dk).
References
Barghoorn, E. S. & Tyler, S. A. Microorganisms from the Gunflint chert. Science 147, 563–577 (1965).
Schopf, J. W. Microflora of the Bitter Springs formation, late Precambrian, central Australia. J. Paleontol. 42, 651–688 (1968).
Gehling, J. G., Droser, M. L., Jensen, S. R. & Runnegar, B. N. Ediacara organisms: relating form to function in Evolving Form and Function: Fossils and Development (ed. Briggs, D. E. G.) 43–66 (Yale Peabody Museum of Natural History, New Haven, 2005).
Bengtson, S. & Zhao, Y. Fossilized metazoan embryos from the earliest Cambrian. Science. 277, 1645–1648 (1997).
Xiao, S., Zhang, Y. & Knoll, A. H. Three-dimensional preservation of algae and animal embryos in a Neoproterozoic phosphorite. Nature 391, 553–558 (1998).
Xiao, S. & Knoll, A. H. Fossil preservation in the Neoproterozoic Doushantuo phosphorite Lagerstätte, South China. Lethaia. 32, 219–238 (1999).
Chen, J. Y. et al. Precambrian animal diversity: putative phosphatized embryos from the Doushantuo Formation of China. Proc. Natl Acad. Sci. 97, 4457–4462 (2000).
Chen, J. Y. The Dawn of Animal World 366 pp (Jiangsu Science Technology Press, Nanjing, China, 2004), [in Chinese].
Bailey, J. V., Joye, S. B., Kalanetra, K. M., Flood, B. E. & Corsetti, F. A. Evidence of giant sulphur bacteria in Neoproterozoic phosphorites. Nature 445, 198–201 (2007).
Huldtgren, T. et al. Fossilized nuclei and germination structures identify Ediacaran ‘animal embryos’ as encysting protists. Science 334, 1696–1699 (2011).
Yin, Z. et al. Sponge grade body fossil with cellular resolution dating 60 Myr before the Cambrian. Proc. Natl Acad. Sci. 112, E1453–E1460 (2015).
Cunningham, J. A., Vargas, K., Yin, Z., Bengtson, S. & Donoghue, P. C. J. The Weng’an Biota (Doushantuo Formation): an Ediacaran window on soft-bodied and multicellular microorganisms. J. Geol. Soc. 174, 793–802 (2017).
Yin, Z., Sun, W., Liu, P., Zhu, M. & Donoghue, P. C. J. Developmental biology of Helicoforamina reveals holozoan affinity, cryptic diversity, and adaptation to heterogeneous environments in the early Ediacaran Weng’an biota (Doushantuo Formation, South China). Sci. Adv. 6, eabb0083 (2020).
Anderson, R. P., McMahon, S., Macdonald, F. A., Jones, D. S. & Briggs, D. E. G. Palaeobiology of latest Ediacaran phosphorites from the upper Khesen Formation, Khuvsgul Group, northern Mongolia. J. Syst. Palaeontol. 17, 501–532 (2019).
Scotese, C. R. Late Proterozoic plate tectonics and palaeogeography: a tale of two supercontinents, Rodinia and Pannotia. Geol. Soc. Lond. Spec. Publ. 326, 67–83 (2009).
Nance, R. D. & Murphy, J. B. Supercontinents and the case for Pannotia. Geol. Soc. Lond. Spec. Publ. 470, 65–86 (2019).
Higgins, A. K., Ineson, J. R., Peel, J. S., Surlyk, F. & Sønderholm, M. Lower Palaeozoic Franklinian Basin of North Greenland. Bull. Grønlands Geol. Unders. 160, 71–139 (1991).
Ineson, J. R. & Peel, J. S. Geological and depositional setting of the Sirius Passet Lagerstätte (Early Cambrian), North Greenland. Can. J. Earth Sci. 48, 1259–1281 (2011).
Bryant, I. D. & Pickerill, R. K. Lower Cambrian trace fossils from the Buen Formation of central North Greenland: preliminary observations. Rapp. Gr.ønl. Geol. Unders. 147, 44–62 (1990).
Peel, J. S. & Willman, S. The Buen Formation (Cambrian Series 2) biota of North Greenland. Pap. in Palaeontol. 4, 381–432 (2018).
Wood, R. et al. Integrated records of environmental change and evolution challenge the Cambrian Explosion. Nat. Ecol. Evol. 3, 528–538 (2019).
Williams, G. E. & Schmidt, P. W. Shuram–Wonoka carbon isotope excursion: Ediacaran revolution in the world ocean’s meridional overturning circulation. Geosci. Front. 9, 391–402 (2018).
Peel, J. S. Spirellus and related helically coiled microfossils (cyanobacteria) from the Lower Cambrian of North Greenland. Rapp. Grønl. Geol. Unders. 137, 5–32 (1988).
Zhang, X.-G. & Pratt, B. R. Middle Cambrian arthropod embryos with blastomeres. Science 266, 637–639 (1994).
Dong, X.-P. et al. Developmental biology of the early Cambrian cnidarian Olivooides. Palaeontology 59, 387–407 (2016).
Dong, X.-P. et al. The anatomy, taphonomy, taxonomy and systematic affinity of Markuelia: early Cambrian to Early Ordovician scalidophorans. Palaeontology 53, 1291–1314 (2010).
Donoghue, P. C. & Dong, X. P. Embryos and ancestors in Evolving Form and Function: Fossils and Development (ed. Briggs, D. E. G.) 81–99 (Yale Peabody Museum of Natural History, New Haven, 2005).
Zhao, Y. & Bengtson, S. Embryonic and post-embryonic development of the Early Cambrian cnidarian Olivooides. Lethaia 32, 181–195 (1999).
Xiao, S., Knoll, A. H., Yuan, X. & Pueschel, C. M. Phosphatized multicellular algae in the Neoproterozoic Doushantuo Formation, China, and the early evolution of florideophyte red algae. Am. J. Bot. 91, 214–227 (2004).
Yin, C., Bengtson, S. & Yue, Z. Silicified and phosphatized Tianzhushania, spheroidal microfossils of possible animal origin from the Neoproterozoic of South China. Acta Palaeontol. Pol. 49, 1–12 (2004).
Yin, L. et al. Doushantuo embryos preserved inside diapause egg cysts. Nature 446, 661–663 (2007).
Xiao, S., Zhou, C., Liu, P., Wang, D. & Yuan, X. Phosphatized acanthomorphic acritarchs and related microfossils from the Ediacaran Doushantuo Formation at Weng’an (South China) and their implications for biostratigraphic correlation. J. Paleontol. 88, 1–67 (2014).
Xiao, S., Hagadorn, J. W., Zhou, C. & Yuan, X. Rare helical spheroidal fossils from the Doushantuo Lagerstätte: Ediacaran animal embryos come of age? Geology 35, 115 (2007).
Long, D. G. F. Kennedy Channel Formation: key to the early history of the Franklinian continental margin, central eastern Ellesmere Island, Arctic Canada. Can. J. Earth Sci. 26, 1147–1159 (1989).
Dewing, K., Harrison, J. C., Pratt, B. R. & Mayr, U. A probable late Neoproterozoic age for the Kennedy Channel and Ella Bay formations, northeastern Ellesmere Island and its implications for passive margin history of the Canadian Arctic. Can. J. Earth Sci. 41, 1013–1025 (2004).
Narbonne, G. M. & Aitken, J. D. Ediacaran fossils from the Sekwi Brook area, Mackenzie Mountains, northwestern Canada. Palaeontology 33, 945–980 (1990).
Knoll, A. H. & Swett, K. Micropaleontology across the Precambrian-Cambrian Boundary in Spitsbergen. J. Paleontol. 61, 898–926 (1987).
Butterfield, N. J. Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 26, 386–404 (2000).
Xiao, S. et al. The Weng’an biota and the Ediacaran radiation of multicellular eukaryotes. Natl Sci. Rev. 1, 498–520 (2014).
Butterfield, N. J. Modes of pre-Ediacaran multicellularity. Precambrian Res. 173, 201–211 (2009).
Knoll, A. H. The multiple origins of complex multicellularity. Annu. Rev. Earth Planet. Sci. 39, 217–239 (2011).
Vidal, G. Giant acanthomorph acritarchs from the upper Proterozoic in southern Norway. Palaeontology 33, 287–298 (1990).
Tiwari, M. & Knoll, A. H. Large acanthomorphic acritarchs from the Infrakrol Formation of the Lesser Himalaya and their stratigraphic significance. J. Himal. Geol. 5, 193–201 (1994).
Grey, K. Ediacaran Palynology of Australia. (2005).
Willman, S. & Moczydłowska, M. Ediacaran acritarch biota from the Giles 1 drillhole, Officer Basin, Australia, and its potential for biostratigraphic correlation. Precambrian Res. 162, 498–530 (2008).
Vorob’eva, N. G., Sergeev, V. N. & Knoll, A. H. Neoproterozoic microfossils from the northeastern margin of the East European Platform. J. Paleontol. 83, 161–196 (2009).
Liu, P. et al. Ediacaran acanthomorphic acritarchs and other microfossils from chert nodules of the Upper Doushantuo Formation in the Yangtze Gorges area, South China. J. Paleontol. 88, 1–139 (2014).
Grazhdankin, D. et al. Doushantuo-Pertatataka–type acanthomorphs and Ediacaran ecosystem stability. Geology 48, 708–712 (2020).
Li, Z.-X., Evans, D. A. D. & Halverson, G. P. Neoproterozoic glaciations in a revised global palaeogeography from the breakup of Rodinia to the assembly of Gondwanaland. Sediment. Geol. 294, 219–232 (2013).
Zhang, S. et al. New paleomagnetic results from the Ediacaran Doushantuo Formation in South China and their paleogeographic implications. Precambrian Res. 259, 130–142 (2015).
Zhou, C. et al. A new SIMS zircon U–Pb date from the Ediacaran Doushantuo Formation: age constraint on the Weng’an biota. Geol. Mag. 154, 1193–1201 (2017).
Matthews, J. J. et al. A chronostratigraphic framework for the rise of the Ediacaran macrobiota: new constraints from mistaken point ecological reserve, Newfoundland. GSA Bull. https://doi.org/10.1130/B35646.1 (2020).
Jiang, G., Shi, X., Zhang, S., Wang, Y. & Xiao, S. Stratigraphy and paleogeography of the Ediacaran Doushantuo Formation (ca. 635–551Ma) in South China. Gondwana Res. 19, 831–849 (2011).
Bowyer, F., Wood, R. A. & Poulton, S. W. Controls on the evolution of Ediacaran metazoan ecosystems: a redox perspective. Geobiology 15, 516–551 (2017).
Pu, J. P. et al. Dodging snowballs: geochronology of the Gaskiers glaciation and the first appearance of the Ediacaran biota. Geology 44, 955–958 (2016).
Acknowledgements
We thank Peter Frykman, Martin Sønderholm, and Jørgen Bøjesen-Koefoed for assistance in the field. We acknowledge financial support from the Danish Natural Science Research Council (FNU Jr. nr. 272-05-0293) for fieldwork in North Greenland in 2006, and funding from the Department of Earth Sciences (Palaeobiology), Uppsala University. Thanks to John Cunningham (University of Bristol), Graham Budd, and Ralf Janssen (Uppsala University) for discussions.
Funding
Open Access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
S.W. and J.S.P. devised the study and interpreted the fossil data. J.R.I. was responsible for interpreting North Greenland geology, the sedimentary log, and the environmental interpretation. N.H.S. performed the initial stable isotope analysis and its interpretation. R.F. performed and interpreted geochemical analysis (major and trace elements, additional stable isotopes). E.J.R. interpreted the geochemical data and wrote the text concerning interpretation of stable isotopes and correlation to the global dataset. All authors contributed to the text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Willman, S., Peel, J.S., Ineson, J.R. et al. Ediacaran Doushantuo-type biota discovered in Laurentia. Commun Biol 3, 647 (2020). https://doi.org/10.1038/s42003-020-01381-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-020-01381-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.